LENS: Regulatory workflows built on context, structure, and real FDA Data , not generic AI responses.
What LENS Does
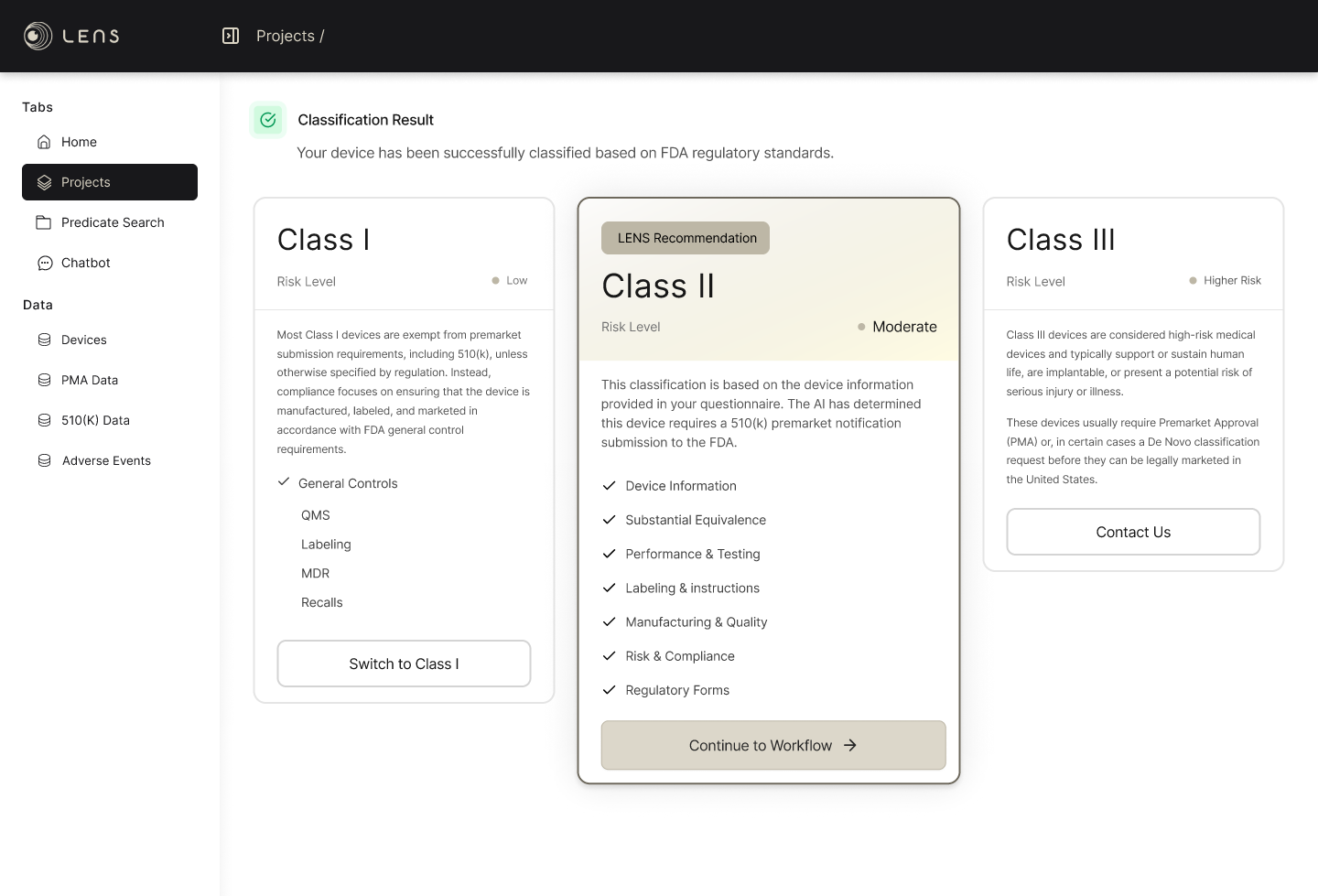
Classifies your device using FDA regulatory logic; not generic AI guesses
Guides you through the exact regulatory pathway for your device class
Generates structured compliance outputs (not essays or advice)
Connects workflows to real regulatory actions, including registration and listing
How LENS Is Different
Workflow-driven, not just a chat-bot: Every answer feeds a regulatory record, nothing gets lost in conversation.
Outputs you can actually use: Checklists, compliance records, and regulatory artefacts, not opinions.
Built by regulatory professionals: Designed to reflect how regulators think, not how AI talks.
Human execution where it matters: When automation stops, experienced regulatory support takes over.
Who LENS Is Built For
Medical device startups and founders
Virtual manufacturers and private-label brands
Engineering-led teams entering regulated markets
Businesses that want clarity before spending £££ on consultants
Regulatory Pathways Supported
-
General Controls compliance workflow
Structured Class I compliance record
FDA Establishment Registration & Device Listing (included)
-
Full 510(k) preparation workflow
Predicate identification and comparison
Performance, labeling, risk, and quality sections
Submission-ready documentation structure
-
Early classification insight
Pathway identification (PMA / De Novo)
Referral to specialist regulatory support